Artificial intelligence (AI) clinical trials use machine learning algorithms and advanced analytics to improve how medical research studies are designed, conducted, and analyzed. The global AI-based clinical trials market reached USD 9.17 billion in 2025, reflecting widespread adoption across pharmaceutical companies and research institutions. These technologies now handle tasks ranging from patient recruitment to data analysis with measurable improvements in efficiency and accuracy.

Source: Grand View Research
The regulatory landscape shifted dramatically in early 2025 when the FDA released comprehensive draft guidance titled "Considerations for the Use of Artificial Intelligence to Support Regulatory Decision-Making for Drug and Biological Products." This framework established clear pathways for AI validation while maintaining patient safety standards. Organizations now have structured approaches for implementing AI systems that meet regulatory requirements across different jurisdictions.
Real-world applications demonstrate significant operational improvements, with some AI systems reducing patient screening time by 42.6 percent while maintaining 87.3 percent accuracy in matching patients to trial criteria. Major pharmaceutical companies report up to 50 percent reduction in process costs through AI-powered document automation. These measurable outcomes have moved AI from experimental technology to essential infrastructure for modern clinical research.
What Are AI Clinical Trials and How They Work
AI clinical trials apply artificial intelligence technology to streamline trial design, speed up patient recruitment, improve retention, and enhance data analysis for faster drug development. Unlike traditional clinical trials that rely heavily on manual processes, AI systems can process thousands of patient records in minutes and predict which trial designs will succeed.
Machine learning algorithms form the backbone of these systems. These are computer programs that learn from data to identify patterns and make predictions. In clinical research, they analyze patient information to spot relationships between factors like genetics, medical history, and treatment responses that humans might miss.
Key AI Components in Clinical Research
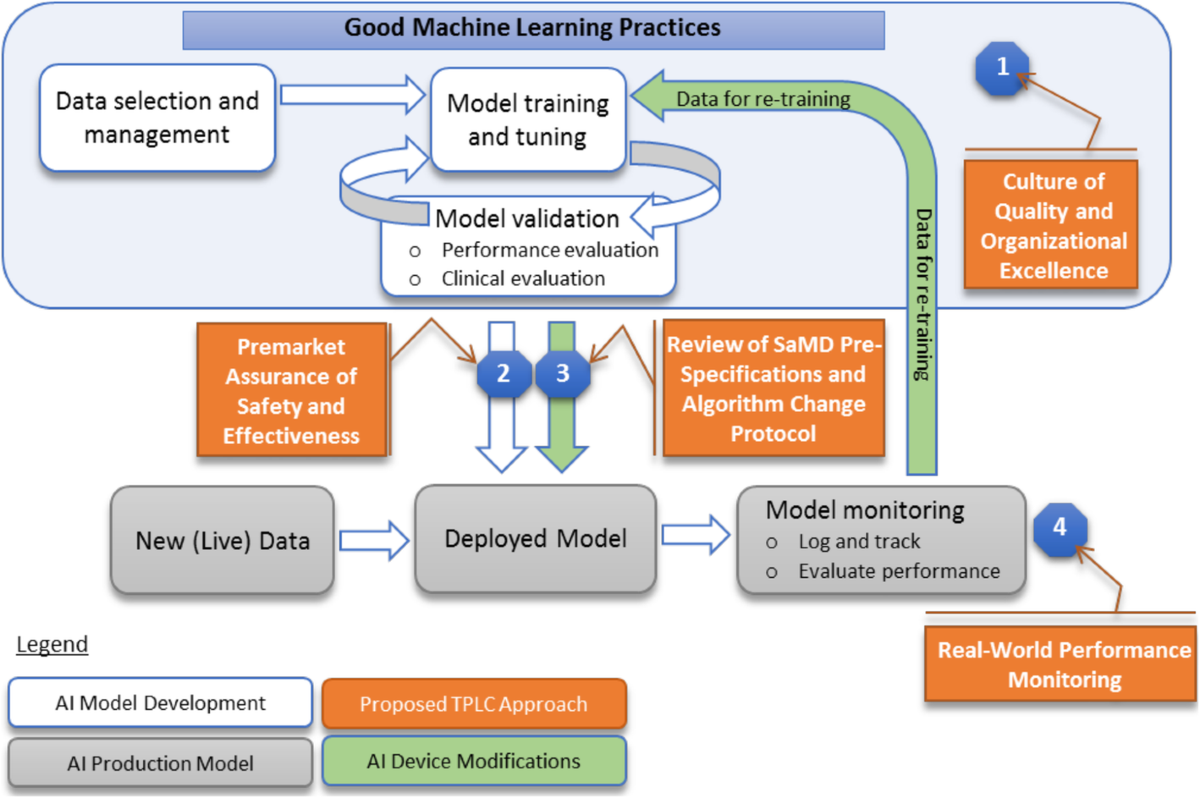
Source: Trials Journal – BioMed Central
Natural language processing (NLP) enables computers to read and understand medical records, research papers, and clinical notes. This technology processes the roughly 80 percent of medical information that exists as unstructured text rather than organized data fields.
Predictive analytics uses statistical methods and machine learning to forecast trial outcomes before studies begin. These systems evaluate patient characteristics, trial design elements, and historical success rates to predict success probability. This approach aligns with predictive AI capabilities that organizations commonly implement for data-driven decision making.
Digital twins create computer simulations that replicate real-world patient populations using mathematical models and data. Researchers can test hypotheses and optimize protocols using virtual patients before conducting studies with real participants.
How AI Transforms Clinical Trial Operations

Source: ResearchGate
AI revolutionizes clinical trial operations by introducing automation, predictive capabilities, and real-time optimization that reshape how research is conducted. The technology addresses core operational challenges through systematic analysis of vast datasets and automated decision-making processes.
Protocol Design and Optimization
AI transforms protocol design by analyzing historical trial data to predict optimal parameters before trials begin. Machine learning algorithms evaluate thousands of variables including patient population characteristics, dosing schedules, and trial duration to identify combinations most likely to succeed.
The technology enables adaptive trial capabilities that modify protocols in real-time based on accumulating data. These systems can adjust dosing levels, patient allocation ratios, or study endpoints as new information emerges during the trial.
Real-Time Data Monitoring
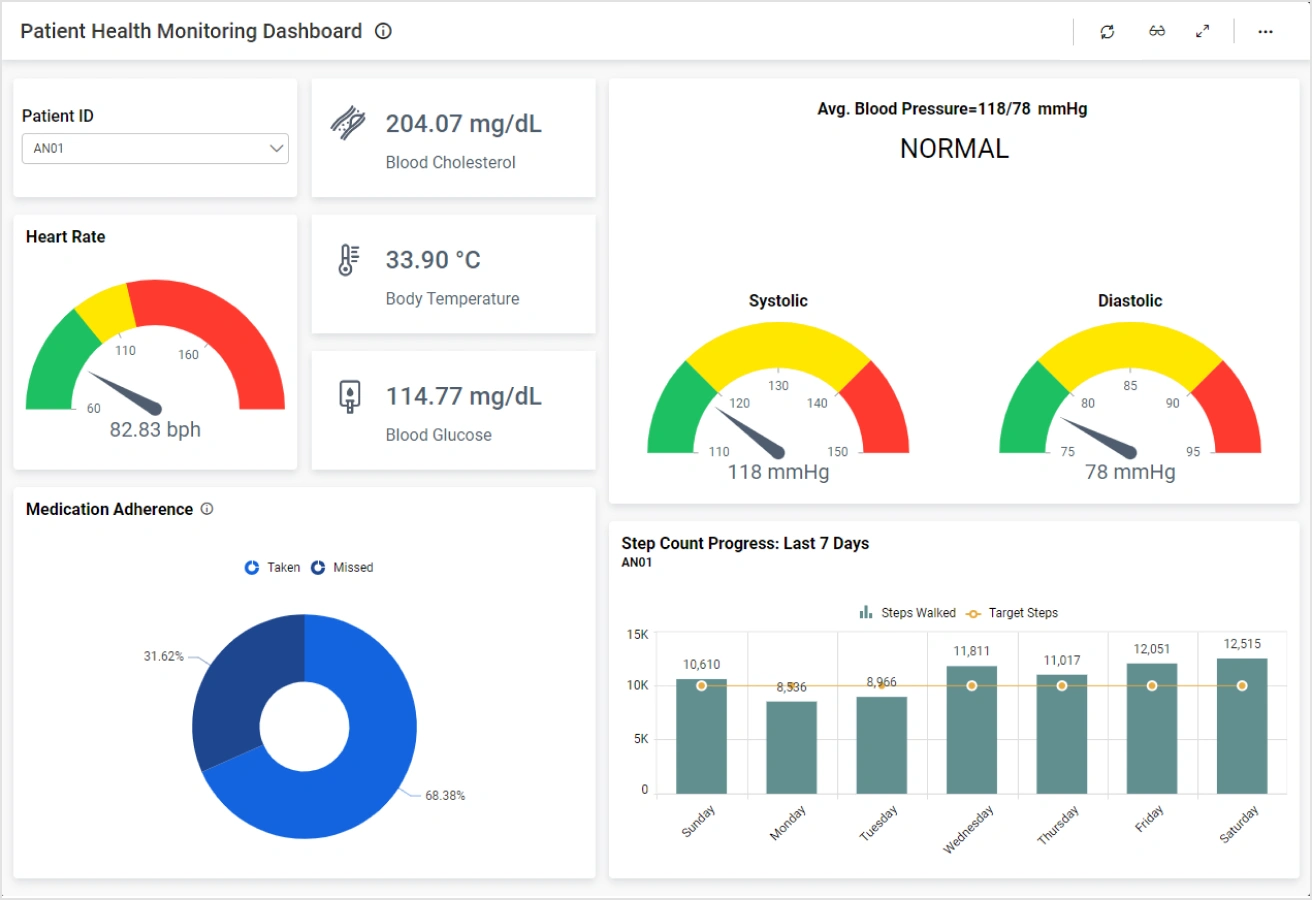
Source: Bold BI
AI provides continuous patient monitoring through automated analysis of data streams from wearable devices, electronic health records, and laboratory results. Safety alert systems powered by AI can detect adverse events as they occur, significantly improving response times compared to traditional periodic reviews.
Real-time visibility includes immediate access to trial progress metrics, enrollment rates, and data quality indicators through automated dashboards. Research teams can monitor key performance indicators and identify potential issues before they compromise trial integrity.
Automated Documentation and Compliance
AI generates required regulatory paperwork by extracting relevant information from trial databases and formatting it according to regulatory standards. Natural language processing algorithms can read clinical data and automatically populate regulatory forms, case report forms, and safety reports.
Compliance documentation automation reduces administrative burden while improving accuracy and standardization of regulatory submissions. AI systems can generate periodic safety reports and regulatory correspondence based on predefined templates and current trial data.
AI Applications for Patient Recruitment and Retention
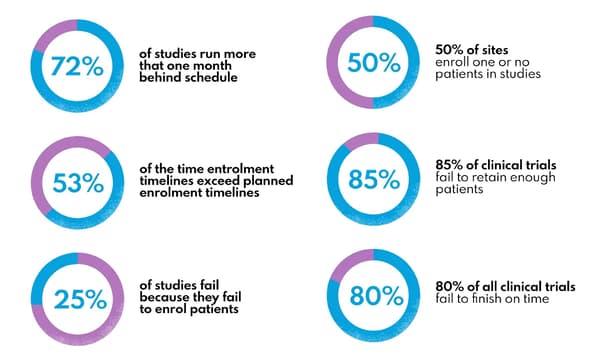
Patient recruitment and retention represent persistent challenges in clinical trials, with studies often taking months longer than planned to enroll sufficient participants. AI addresses these challenges through predictive models that identify suitable candidates faster and personalized approaches that keep participants engaged.
Electronic Health Record Screening
AI systems scan electronic health records to identify potential clinical trial candidates by analyzing medical histories, diagnostic codes, laboratory results, and treatment patterns. Natural language processing technology extracts relevant information from unstructured clinical notes that traditional database searches cannot access.
The automated screening process evaluates thousands of patient records simultaneously against specific trial criteria, identifying potential candidates in minutes rather than weeks. Advanced screening capabilities extend beyond basic eligibility to assess patient likelihood of successful trial completion.
Predictive Patient Matching
Algorithmic matching systems analyze genetic markers, biomarker profiles, and comprehensive medical histories to predict which patients will respond well to specific treatments. These algorithms consider factors like genetic polymorphisms, protein expression levels, and previous treatment responses.
TrialGPT and similar specialized AI models can process complex medical information to provide individual criterion assessments and consolidated predictions for trial suitability. The matching process extends beyond basic eligibility criteria to predict patient likelihood of completing the trial successfully.
Digital Outreach Strategies
AI-powered communication platforms create personalized outreach campaigns by analyzing patient demographics, communication preferences, and health literacy levels. These systems optimize message timing, communication channels, and content personalization to maximize response rates.
Digital marketing algorithms identify individuals through social media platforms and search behavior analysis, enabling targeted recruitment approaches that reach patients actively seeking treatment options. Automated engagement systems maintain ongoing communication through chatbots and personalized email campaigns.
Retention Risk Assessment
Predictive analytics models analyze participant behavior patterns, appointment attendance, and medication compliance to identify patients at high risk of dropping out. Early warning systems monitor factors like missed visits and delayed survey responses to generate risk scores.
Machine learning algorithms process multiple data streams to identify subtle patterns that indicate declining engagement. Intervention tactics include personalized reminder systems, modified visit schedules, and targeted education materials addressing specific patient concerns.
Benefits of Implementing AI in Clinical Research

Source: ResearchGate
AI delivers measurable improvements across clinical research operations, transforming how pharmaceutical companies conduct trials. The technology generates substantial return on investment through enhanced efficiency, improved quality, and accelerated timelines.
Cost Reduction and Operational Efficiency
AI automation eliminates time-intensive manual work across clinical trial operations. Medical coding systems save approximately 69 hours per 1,000 terms coded while achieving 96 percent accuracy compared to human experts. These systems reduce administrative burden while maintaining quality standards.
Resource optimization occurs through intelligent allocation of personnel and materials based on predictive analytics. AI algorithms forecast demand patterns and manage inventory distribution, preventing supply shortages while minimizing waste.
Improved Data Quality
AI reduces human error through automated data validation and quality control systems that identify inconsistencies more reliably than manual review processes. Machine learning algorithms detect subtle patterns that might indicate data quality issues.
Data reconciliation tools automatically cross-check information from multiple sources and flag discrepancies for review. These systems maintain consistent performance standards without fatigue or attention lapses that can affect human data review processes. Proper AI data management becomes crucial for maintaining data integrity throughout the trial lifecycle.
Accelerated Development Timelines
AI shortens drug development cycles through faster patient recruitment and trial execution. Predictive analytics platforms reduce screening time by 42.6 percent while maintaining 87.3 percent accuracy in matching patients to trial criteria.
Trial design optimization occurs through machine learning simulations that predict outcomes and refine protocols before implementation. Adaptive trial designs modify parameters in real-time based on interim results, improving efficiency while maintaining statistical validity.
FDA Regulations and Compliance Requirements
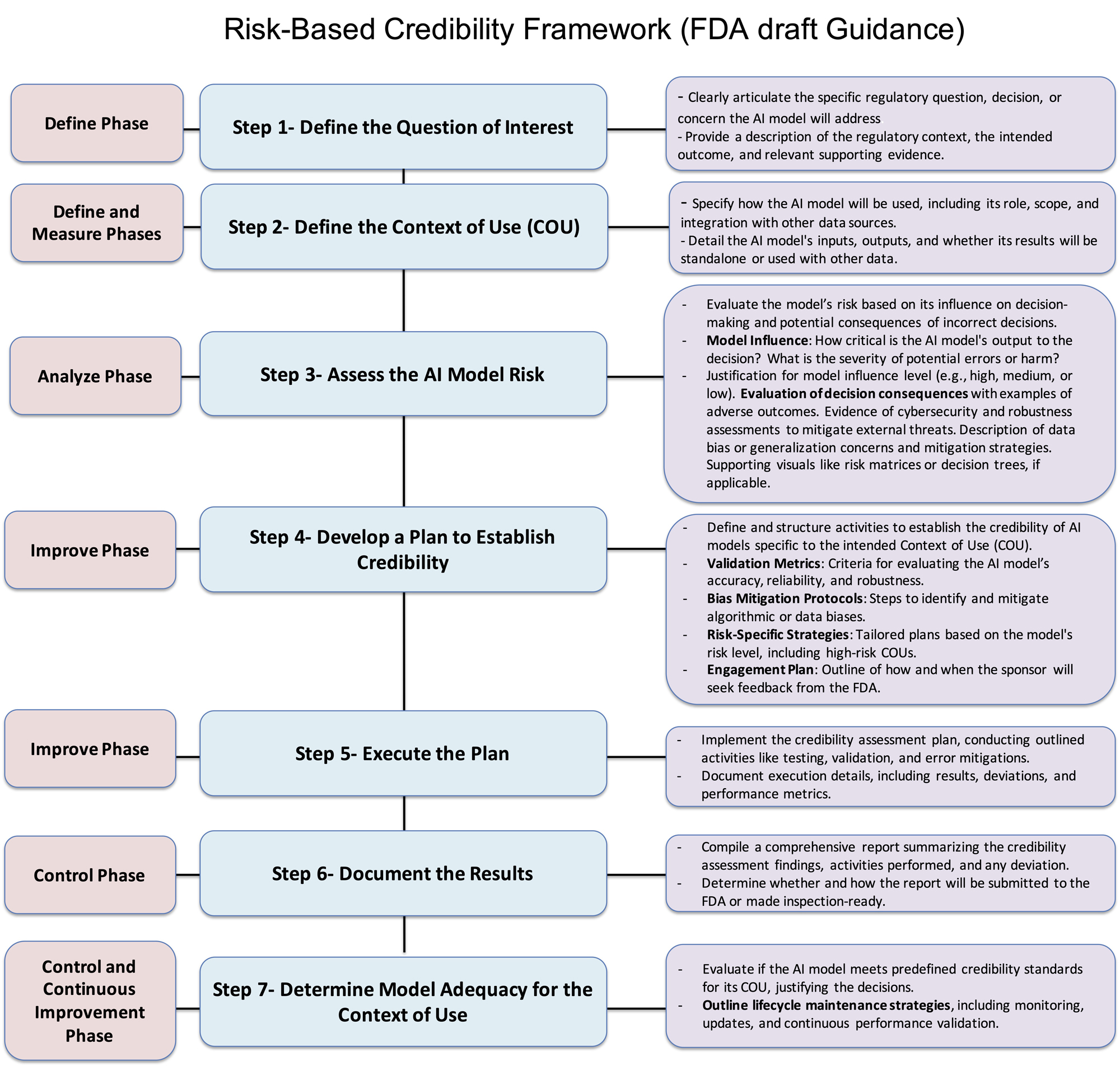
Source: Bioprocess Online
The FDA established comprehensive regulations for AI in clinical trials through their January 2025 draft guidance. This framework provides the first systematic approach for evaluating AI models across two critical dimensions: model influence and decision consequence.
Risk-Based Assessment Framework
The FDA categorizes AI models into three risk levels based on their potential impact on patient safety and trial outcomes:
- Low-risk applications — Basic data organization and administrative functions with minimal clinical impact
- Medium-risk applications — Decision support tools that influence but don't directly determine clinical actions
- High-risk applications — AI systems that directly impact patient safety or primary efficacy endpoints
Risk assessment requires comprehensive evaluation across multiple dimensions. Model influence measures how much AI outputs affect clinical decision-making processes. Decision consequence assesses potential negative outcomes from incorrect AI determinations.
Validation Requirements
AI system validation requires comprehensive documentation extending beyond traditional software validation protocols. Organizations must document training dataset characteristics including size, diversity, representativeness, and bias assessment results.
Model architecture documentation includes algorithm selection rationale, parameter optimization procedures, and performance benchmarking results. Validation studies must demonstrate AI system performance across accuracy metrics, reliability testing, and generalizability studies. Organizations should establish AI management standards to ensure consistent validation processes.
Transparency and Explainability
AI systems used in clinical trials must provide interpretable outputs that healthcare professionals can understand and validate. Explainability requirements mandate implementation of technical approaches that identify key contributing features to AI predictions.
Algorithmic transparency documentation includes detailed descriptions of how AI models process input data and generate outputs. User interfaces must present AI outputs in formats that enable clinical professionals to evaluate recommendations effectively.
Implementation Challenges and Solutions
Organizations face significant obstacles when implementing AI in real-world healthcare environments. AI models frequently underperform when deployed outside controlled testing environments due to factors not adequately addressed during development phases.
Common Implementation Barriers
Technical integration barriers arise when legacy systems lack standardized data formats and modern APIs required for AI connectivity. Resource constraints often emerge when organizations underestimate computational power, storage, and bandwidth requirements for AI systems.
Performance degradation occurs when AI models experience accuracy decline when applied to populations different from training data. Workflow disruption represents a critical challenge as existing clinical processes require substantial modification to accommodate AI-driven decision-making.
Addressing Bias and Fairness
AI systems can perpetuate existing biases present in training data, leading to unfair treatment of certain patient populations. Studies document concerning cases where AI diagnostic tools showed reduced accuracy for specific demographic groups compared to others.
Organizations address bias through comprehensive data audit processes that examine training datasets for demographic representation. Fairness testing methods evaluate AI performance across different population subgroups to identify performance gaps before deployment.
Change Management Strategies
Healthcare professionals require new skills and modified workflows to effectively use AI-powered tools in clinical practice. Comprehensive change management approaches must address both technical aspects and practical integration strategies.
Training programs address technical aspects of AI system operation and practical integration strategies for clinical workflows. Physician engagement strategies recognize that clinical staff acceptance is critical for AI success, involving clinicians in system selection and customization processes. Organizations can benefit from establishing an AI Center of Excellence to coordinate implementation efforts across departments.
Future Trends and Market Outlook
The AI clinical trials market experienced significant expansion in 2025, growing from $7.73 billion in 2024 to $9.17 billion. Financial analysts project the market will reach $21.79 billion by 2030, representing sustained confidence in AI technologies for clinical research.
Technology Advancements
Generative AI creates comprehensive trial protocols through automated document generation that transforms protocol development from manual approaches to systematic, evidence-driven methodologies. These systems analyze vast databases of successful trial designs and regulatory requirements.
Wearable devices and Internet of Things integration create comprehensive remote monitoring ecosystems that collect continuous patient data throughout clinical trials. AI algorithms process these continuous data streams to identify patterns that traditional periodic assessments might miss.
Investment Patterns
Venture capital investment has shifted toward companies with demonstrated commercial traction rather than purely technical capabilities. Investors prioritize revenue-generating AI applications over broad-platform approaches requiring extensive customization.
Corporate venture capital arms of pharmaceutical companies increase activity through strategic partnerships that provide access to innovative technologies. These investments often include collaboration agreements enabling pharmaceutical companies to pilot AI technologies within their trial portfolios.
Frequently Asked Questions About AI Clinical Trials
How long does implementing AI in clinical trials typically take?
Source: LinkedIn
AI clinical trial implementation typically takes six months to two years, depending on system complexity and organizational readiness. Simple applications like patient recruitment algorithms can be deployed in six to nine months when organizations have adequate infrastructure. Comprehensive AI systems that integrate across multiple trial operations require 18 to 24 months for full implementation including validation and training phases.
What specific costs should organizations expect for AI clinical trial technology?
Source: nexocode
Budget allocation varies significantly based on trial scope and technology sophistication. Small-scale AI tools for patient recruitment typically require investments of $50,000 to $200,000 per trial. Comprehensive AI platforms can cost $500,000 to $2 million annually for enterprise-wide implementations across multiple trials. Cloud-based AI services with pay-per-use models provide alternatives, with costs typically ranging from $10,000 to $50,000 per trial.
Which AI applications show results fastest in clinical trials?
Patient recruitment algorithms and data monitoring systems typically deliver the fastest results due to their immediate operational impact and straightforward integration requirements. These applications can show measurable improvements within three to six months of deployment. Medical coding automation also provides rapid returns, with organizations reporting time savings of 69 hours per 1,000 medical terms processed.
How do pharmaceutical companies evaluate AI vendors for clinical trials?
Vendor evaluation prioritizes regulatory compliance experience, system integration capabilities, and proven clinical trial expertise. Organizations assess vendors based on FDA guidance compliance, validation documentation quality, and experience with similar therapeutic areas. Technical evaluation criteria include data security measures, system scalability, and integration capabilities with existing clinical trial management systems.
AI clinical trials represent a fundamental transformation in medical research, moving from experimental technology to essential infrastructure that delivers measurable value. The regulatory frameworks established in 2025 provide the foundation for responsible AI adoption while maintaining scientific rigor. Organizations that approach AI implementation with clear strategic vision and comprehensive validation procedures will be best positioned to leverage these technologies for improving clinical trial outcomes.



